Fick's Law
Fick's law states that local differences in solute concentration will result in a net flux of solute molecules moving from high concentration regions to low concentration regions.
The Waterloo Emitter™ emits amendment materials (gas or liquid) via a diffusion mechanism in accordance with Fick's first law. The Waterloo Emitter may be described as a Fickian system whereby a polymeric membrane (silicone or LDPE tubing) separates a zone of higher internal concentration (gas or liquid inside the tubing) from a zone of lower external concentration of the same species in the groundwater.
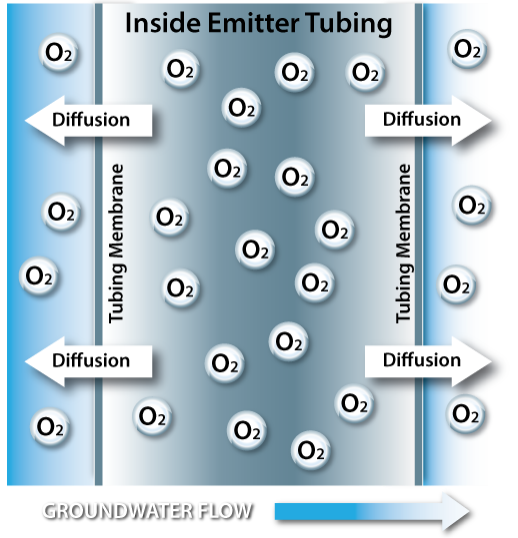
In other words, when an amendment is introduced into the Waterloo Emitter tubing, a concentration gradient is set up between the inside of the tubing and the groundwater. As such, the amendment diffuses across the tubing membrane from the higher concentration inside the tubing to the lower concentration in the groundwater.
In mathematical terms, the net amount of material diffusing across a unit cross-section (J, flux) perpendicular to a membrane of known thickness (x) is proportional to the change in concentration (C, higher concentration less lower concentration) divided by the thickness of the membrane.
At steady state, Fickian diffusion can be approximated by:
J = D (C Waterloo Emitter – C groundwater) / membrane wall thickness,
where D is the diffusion coefficient for the membrane material and is expressed in L2 / t (e.g. cm2/s).
Diffusion coefficient for Waterloo Emitter silicone tubing is 6.7E-07 cm2/s
Diffusion coefficient for Waterloo Emitter LDPE tubing is 1.73E-08 cm2/s |
|
Practical implications for use of the Waterloo Emitter™:
- The net flux of liquid or gaseous amendment across the diffusive tubing will be dependent on the background level of amendment (e.g. dissolved oxygen) in groundwater. Therefore, lower background groundwater concentrations will result in a higher flux from the Waterloo Emitter™
- For liquid amendments, the net flux of liquid amendment across the diffusive tubing will be dependent on the concentration of the liquid amendment used. Therefore, higher concentrations correspond to a higher chemical gradient which will result in a higher flux from the Waterloo Emitter
- For gaseous amendments, the net flux of gaseous amendment across the diffusive tubing will be dependent on the pressure (which is proportional to the concentration) within the diffusive tubing of the Waterloo Emitter. Therefore, higher pressure will result in a higher flux from the Waterloo Emitter ™)
- In gas release applications, mass is released from the tubing on a molecular basis and immediately dissolves in the groundwater thus there are significantly reduced mass transfer limitations compared to commonly used gas sparging (bubbling) techniques
- Diffusion will occur until there is equilibration inside and outside of the tubing. With the Waterloo Emitter, the amendment can be replenished constantly and, since groundwater flow around the Emitter is continuous, equilibration is never reached. This results in steady, controlled diffusion into the groundwater, without any decrease in concentration due to bubbling
- When oxygen is used as the amendment gas, it provides a regulated supply of dissolved oxygen that is critical to the proper growth and maintenance of a natural in-situ microbial population. This allows for enhanced aerobic bioremediation of contaminated groundwater.

|




